Sign up for our newsletter!
Your data will be handled in compliance with our privacy policy.
Your data will be handled in compliance with our privacy policy.

During the festive season, there is time for longreads. We, therefore, want to offer a text that goes beyond what we usually write here on the blog but is not entirely unrelated to what Smoltek does. This is the story of the origin of the capacitor. Happy reading!
Thomas Barregren • December 24, 2023
Like all capacitors, ours originates from the Leyden jar, a glass bottle that can store electrical charge. Ewald Georg von Kleist was the first to experience this ability when he received a severe electric shock in October 1745. Pieter van Musschenbroek followed suit when he repeated the experiment a few months later, in January 1746. This is the story about their shocking discovery and the early development of the capacitor – a groundbreaking component that is ubiquitous in today’s electronics.
If you prefer to listen, you can click the play button below to hear the article read aloud.
The first known observation of what we now call static electricity was made by the Greek philosopher Thales of Miletus in 600 BC. He noted that amber, when rubbed against cloth, attracts light objects such as hairs. But it took more than two thousand years before anyone set out to explore this force.
Sir William Gilbert’s magnum opus – De Magnete, published in 1600 – contains some of the earliest systematic studies of electricity. He observed that substances like glass, sulfur, and diamond exhibited the same attraction property as amber when rubbed. He called this an electric force. The name is derived from the Greek word for amber.
Six decades later, Otto von Guericke invented a simple machine to generate static electricity. His electrostatic generator consisted of a ball of sulfur cast on an iron axle, which, when pulled around and subjected to friction, gave off electric charges, which manifested themselves in sparks.
In 1705, Francis Hauksbee created an improved electrostatic generator. It consists of a crank that rotates a large wheel from which a belt runs to a smaller wheel attached to an axle through a glass ball.
At the beginning of the 1730s, Charles François de Cisternay du Fay, also known as just Dufay, discovered the existence of two types of electrical charges. He named them vitreous and resinous after the material he used to produce them (glass and resin), but we call them positive and negative. Du Fay also noticed that two of the same repel and two of the opposite attract each other. Moreover, he differentiated materials in electrics and non-electrics, which are similar but not identical to what we today call conductors and insulators.
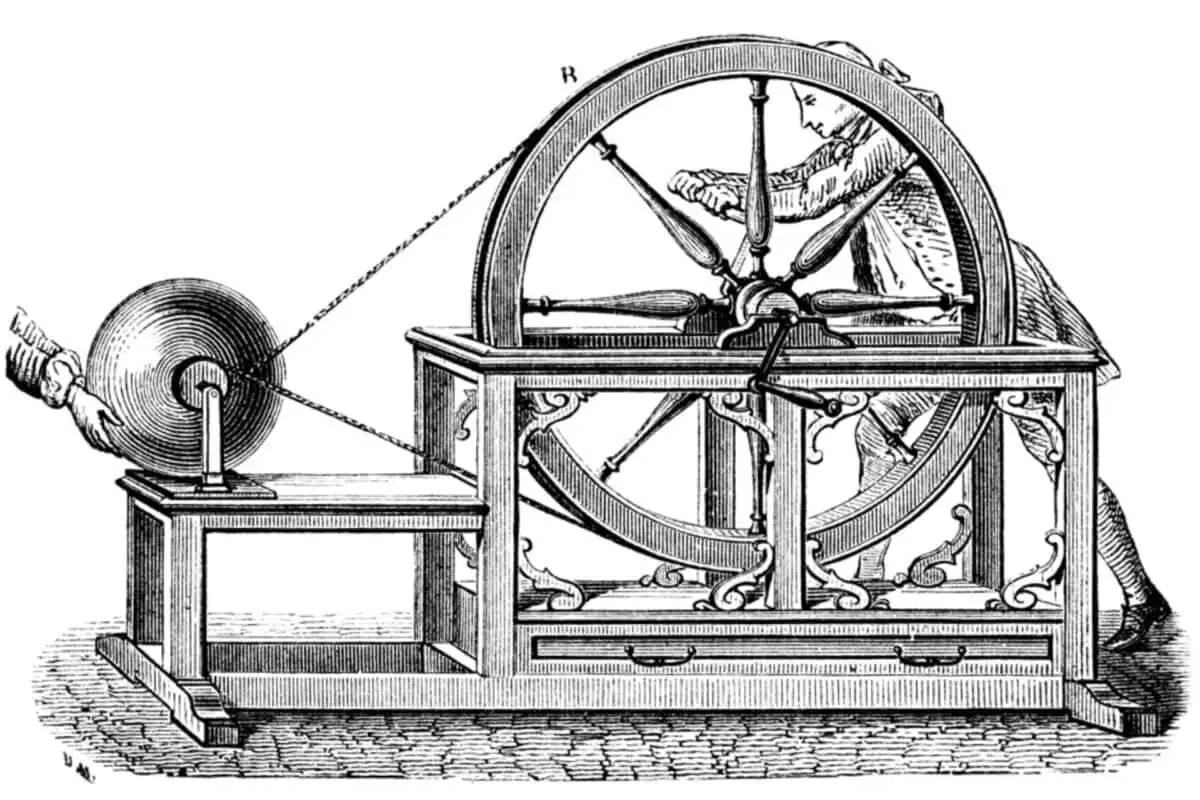
In the fall of 1745, things sparked the exploration of electricity.
First up was Matthias Bose, who makes a name for himself as a flamboyant demonstrator of experiments with static electricity. In one of his most famous tricks, he ignites alcohol floating on top of the water by generating static electricity, which he conducts through a metal bar to the water.
His main contribution to this story is the use of the metal bar. He hung it horizontally with one end above the Hauksbee’s rotating glass ball. If the distance is not too great, or if a metal chain hangs from the metal bar down to the glass ball without touching it, the metal bar will capture static electricity that can then be transferred to something else. In Bose’s show, it was the water with alcohol on the surface.
Next up was Ewald Georg von Kleist. Inspired by Bose’s metal bar, he tried to prove that electricity can be understood as a fluid.
On October 11, 1745, he filled a small medicine bottle with alcohol and closed it with a cork through which a nail was inserted. He then used a metal bar to transfer static electricity from an electrostatic generator to the nail. In this way, he imagined that static electricity was poured into the alcohol.
Von Kleist knew that the glass is an insulator. Therefore, he was convinced that static electricity could be “captured” and retained in the bottle.
He accidentally touched the nail.
Zap!
He was thrown across the room.
Von Kleist had received a strong electric shock, proving that he had captured electricity in the bottle (but not in the way he thought). In fact, he had created the world’s first capacitor.
Von Kleist wrote about his experiment to several other electrical experimentalists. Some wanted to try it themselves.
Warned by Von Kleist’s example, they kept their distance when the experiment was repeated. This proved unnecessary, as the experiments failed, and nothing happened.
What a disappointment.
Von Kleist didn’t write to Anderas Cunaeus, a lawyer and amateur scientist. Yet Cunaeus came up with something strikingly similar. Maybe he had heard about Von Kleist’s experiment. Or not. Nevertheless, he did a similar experiment with household items at his home.
The result?
Zap!
Cunaeus was out for two full days.
It is a cold evening in January 1746. Snow is falling thickly in the courtyard of Leiden University – the oldest in the Netherlands. A few tardy students are crossing the courtyard from a lecture hall to the evening’s supper. They pass by the laboratory window of Pieter van Musschenbroek.
Professor van Musschenbroek stands at the window, thinking of his friend, the lawyer Anderas Cunaeus, who has done what professionals have failed to do – recreate von Kleist’s experiment from three months ago. Now, he will try to repeat the experiment himself.
He adjusts the chain so that its free end comes as close as possible to the glass ball without touching it.
Meanwhile, one of his disciples hangs a metal wire over the other end of the metal rod. He then fills a glass jar with water.
They are now ready for the experiment.
While one of the students cranks the wheels of the electrostatic generator, Professor van Musschenbroek takes the water-filled glass jar with his bare hands and holds it up so that the metal wire is lowered into the water.
Another student now takes clothes in his hands and holds them against the rotating glass ball. The friction between the glass and the cloths creates static electricity, passing to the metal bar through the metal chain and further to the water through the metal wire.
While standing there, he thinks about Bose. He may be an assiduous self-promoter, but storing and transferring static electricity with a metal bar was pretty clever.
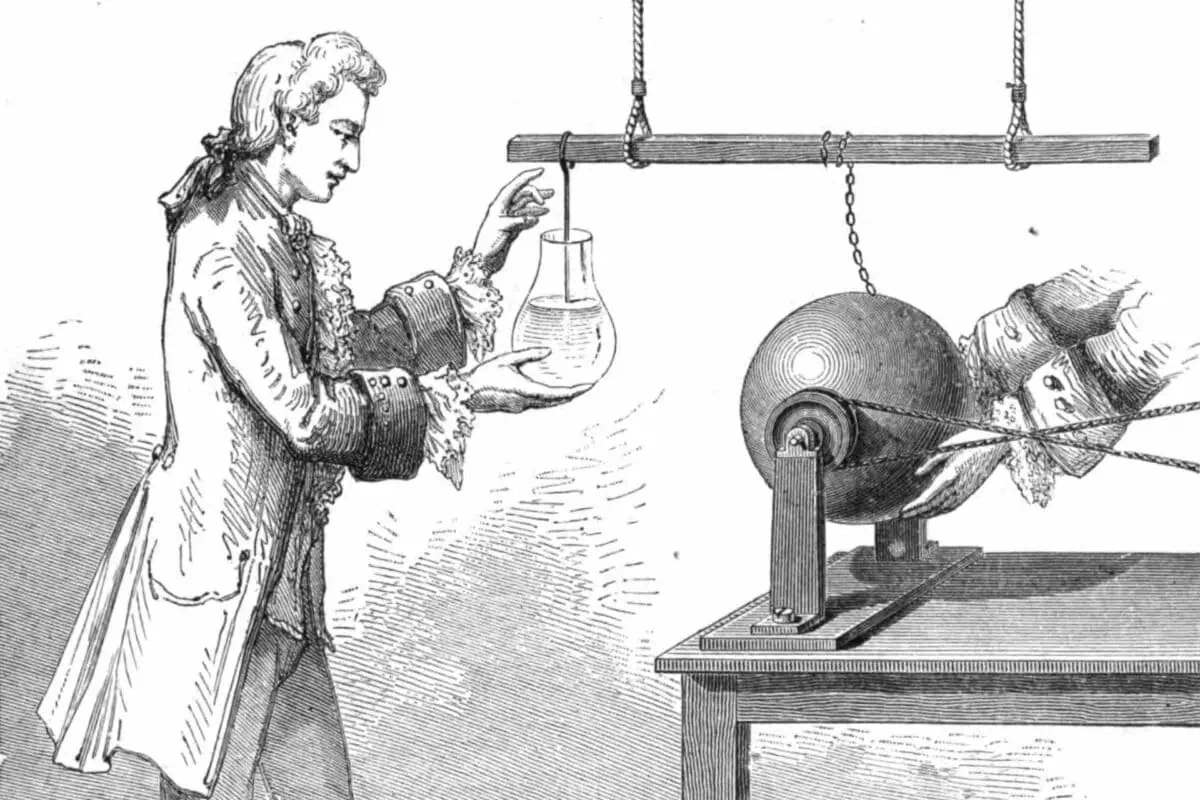
It’s time for the experiment itself. Professor van Musschenbroek reaches out with his left hand to the metal wire hanging into the glass jar he holds in his bare right hand.
Zap!
On January 20, 1746, Professor Musschenbroek wrote to his designated contact at the Paris Academy and told him about the experiment. He began his letter:
I would like to tell you about a new but terrible experiment, which I advise you never to try yourself, nor would I, who have experienced it and survived by the grace of God, do it again for all the kingdom of France.
Pieter van Musschenbroek, January 20, 1746
Abbé Jean-Antoine Nollet confirmed the experiment and then read Musschenbroek’s letter at a public meeting of the Paris Academy in April 1746. He named the electrical storage device Leyden jar, after Professor Musschenbroek’s university.
Professor van Musschenbroek realized that a condition for the experiment to succeed was that there was a conductor connected to earth on the outside of the glass jar.
In the cases of von Kleist, Cunaeus, and himself, they were the conductor to earth, as they held the glass jar with their bare hands.
Those who failed had put down the glass bottle for fear of an electric kiss. (In all honesty, they followed the best practices of their time and deliberately ensured that the glass jar was not grounded).
The Leyden jar did not only shock Musschenbroek. Society was also shocked – both literally and figuratively.
Self-proclaimed “electricians” held public demonstrations where they gave sparkling shows and jolted their audience. Natural philosophers electrocuted animals to better understand this new force. Physicians applied electric shocks to humans to cure various ailments. And technologists sent charges through wires over rivers and lakes to figure out what it could be used for.
The news of Leyden jar’s ability to store electrical charge made its way across the pond to what, for a few more years, would only be referred to as the American Colonies. There, Benjamin Franklin experimented with Leyden jars.
It is 1748, and we are at the home of Benjamin Franklin in Philadelphia. He has just filled a Leyden jar with charge and put it on a glass insulator.
With a look of determination, he begins his experiment.
Franklin pulls out the cork of the jar and lifts it with the wire through it. He grasps the bottle with one hand, and brings a finger of the other hand near its mouth. A strong spark comes from the water, as painful as if he had touched the wire before removing it. It convinces Franklin that the electric charge is not in the wire. It is still in the jar.
He pours water from the charged jar into an empty second jar. The second jar shows no sign of electric charge. Thus, the electric charge must remain in the now empty first jar.
“What the frock!” Franklin exclaims.
He reaches for the teapot containing fresh, unelectrified water and pours new water into the first jar. Testing it again, he finds it still capable of giving him a jolt.
He later writes:
Thus the whole Force of the Bottle and Power of giving a Shock, is in the Glass itself; the Non-electrics in Contact with the two Surfaces serving only to give and receive to and from the several Parts of the Glass; that is, to give on one Side, and take away from the other.
Benjamin Franklin
Franklin now asked whether the shape of the jar is crucial to its ability to store charge.
He took a piece of window glass and put it in his hand to test this. On top of it, he then puts a plate of lead that he had electrified.
Now comes the test itself: He puts a finger to the plate. Zap! There was a spark and shock. In other words, the shape doesn’t matter.
But where is the charge stored? On the glass? Or on the hand and the lead plate in contact with the glass?
Franklin placed a piece of window glass between two lead plates to find out. The whole stack rests in his hand while he electrifies the top plate.
He then separates the parts. The glass plate gave off tiny stinging sparks when he touched it. He could feel this in many places on the glass surface. He also notes that there are no charges in the lead plates. Finally, he returned the glass between the lead plates.
Now, the moment of truth: Franklin grabs both lead plates. Zap! A strong jolt showed that the charge was still there.
From this experiment, Franklin concludes that the electric charge was on the glass and that the lead plates only served to bring the charge to or from its surface.
Franklin was not the first to discover that water is unnecessary and a glass plate works just as well as a glass jar to hold a charge. John Bevis had already demonstrated this in the same year. However, Franklin did not find out until later.
But unlike Bevis, who thought that the charge was in the metal in touch with the glass plate, Franklin proved that the charge is actually on the surface of the glass.
Moreover, Franklin also figured out that there are not two types of charges, as du Fay had stated almost two decades earlier, but only one charge: the negative one. A positive charge arises when a negative charge is removed.
In other words, instead of seeing positive and negative as two separate entities, Franklin viewed them as two states: the presence of a negative charge and the absence of it.
With these insights, gained just a few years after the discovery of the Leyden jar, it was now possible to explain what happened on that snowy winter evening when Professor van Musschenbroek had himself electrified.
The friction against the glass ball generates positive charges. These are passed through the chain, bar, and wire into the water.
Since equal charges repel each other, the positive charges are pushed against the inside of the wall of the glass jar. Since the glass is an insulator, the charges cannot escape the jar.
But the force with which charges repel equal charges and attract opposite charges isn’t stopped by an insulator. Therefore, the positive charges inside the glass jar repel negative positive outside the glass and attract negative charges.
In its natural state, the glass’s exterior has an equal mix of positive and negative charges. However, the positive charges inside the glass repel the external positive charges, redistributing them away from the surface while attracting negative charges closer, concentrating them.
This process requires a ground path for the displaced positive charges. This is where the hand becomes crucial, acting as a conductor to complete the circuit and allow these charges to reach the ground through the experimentalist’s body.
As the positive charges in the jar grow, so does the resistance that newly added positive charges must overcome. Eventually, the jar reaches its maximum charge capacity. There are a large number of positive charges on the inside of the glass and an equal number of negative charges on the outside of the glass.
The charges remain as long as there is no way for the positive charges to get to the negative ones. So you could say that von Kleist was right – the jar stores charge, but not in the liquid, as he thought, but on the outside and inside of the jar.
But as soon as there is an opportunity for the positive charges to get to the negative ones, they will take it. This is what happened to von Kleist, Cunaeus, and Professor van Musschenbroek when they touched the nail or chain that was in contact with the water when they held the jar. The positive charges rushed through their poor bodies – from the hand touching the nail or chain to the hand holding the jar. Zap!
The above is a modern description of how a Leyden jar works, using knowledge from the mid-18th century. It is a simplified view of, in particular, what happens on the surface and inside the glass wall.
It wasn’t until the 1910s that scientists had the knowledge to understand what happens at the subatomic level. It’s pretty damn interesting stuff, and the story leading up to it is at least as exciting as the one we’ve heard so far. But telling this story would take us on too many winding side roads, and dwelling on the capacitor’s inner workings is another article, so let’s fast-forward the timeline to the beginning of the 20th century.
Did you see what I just did? I used the word capacitor in the context of the Leyden jar. That’s because a Leyden jar is actually a capacitor. That makes von Kleist’s medicine bottle the very first ever made.
Furthermore, when Franklin put metal on both sides of a piece of window glass, he created the world’s first parallel plate capacitor.
In fact, two parallel plates insulated from each other are the very essence of a capacitor. The insulation does not need to be made with glass. It can be vacuum, air, or any material that doesn’t allow charges to move across the gap between the two plates.
Some isolators, like glass, have the property that they increase the ability of the capacitor to store charge thanks to a phenomenon called polarization (subject of another article). Such an isolator is called a dielectric.
All this began to be understood in the 19th century and led to the first modern capacitor.
The Leyden jar is a high-voltage capacitor. With the development of telegraphy, telephones, and radio in the late 19th century, there was a need for smaller capacitors for lower voltages. This accelerated the pace of innovation.
The first modern capacitor was developed by D. G. Fitzgerald. It consisted of metal foil with impregnated paper as a dielectric. He patented the solution in 1876. Paper capacitors, as they came to be known, were further developed and widely used throughout the 1950s when plastic film capacitors began to appear.
The first paper capacitors were followed by a formidable explosion of different types of capacitors:
Current and future demands for extreme miniaturization or for ultra-reliable and ultra-stable service result in the development of new types of capacitors.
Integrated capacitors are capacitors formed by appropriate metallization patterns on an isolating substrate. These include metal-oxide-metal (MOM) capacitors, metal-oxide-semiconductor (MOS) capacitors, and metal-insulator-metal (MIM) capacitors. Despite the name of these types of capacitors, they can be encapsulated and sold as regular, although very tiny, capacitors. Sometimes, they are also called silicon capacitors since the substrate is usually silicon. Silicon compounds can also be used as dielectrics.
Deep trench capacitors (DTCs) are created on a semiconductor substrate by creating deep recesses, called trenches, to maximize the surface area and capacitance in a small footprint. DTCs are also known as trench silicon capacitors (TSC) and silicon capacitors (SiCap).
Glass capacitors are modern Leyden jars. They consist of multiple layers of metal intertwined with glass, similar to how MLCCs are built. They are used in the most extreme situations. Glass capacitors are the most durable capacitors in all respects. For example, they can withstand high doses of nuclear radiation and strong neutron radiation.
Note that the term silicon capacitors can be used for both integrated capacitors and deep trench capacitors. Quite confusing.
Of course, we can’t write an article without talking about our carbon nanofiber fiber metal-insulator-metal (CNF-MIM) capacitor.
Without going into detail, we can say that CNF-MIM capacitors are produced in much the same way as the integrated capacitors of the MIM type. You might have guessed this by the name.
However, they differ from MIM capacitors in one fundamental way where CNF-MIM capacitors are more similar to DTC. Both DTC and CNF-MIM capacitors use nanotechnology to increase the surface area. This is important because the ability to store charges is directly proportional to the area.
But the area can only increase so much for DTC; the sky’s the limit for CNF-MIM capacitors (almost literally). DTCs have trenches that are limited how deep they can go before the substrate becomes too brittle and breaks. CNF-MIM capacitors, on the other hand, build carbon nanofibers on top of the substrate.
Pieter van Musschenbroek was not the first to discover the Leyden jar. Still, he was the one who made it known and sparked a flurry of research into the nature of electricity. Electricity was studied frantically for the next hundred and sixty years or so. It’s an incredibly fascinating story, but since we’re approaching this article’s end, we’ll leave it at that.
Fast forward to today, Smoltek is driving the development of successors to the Leyden jar. The historical perspective makes us feel humble. Sure, our CNF-MIM technology is a big step forward for capacitors, but it has been preceded by other, more essential steps made by others before us. We feel happy to be a small link at the end of this 275-year-long chain of discovery and development.
Zap!
Your data will be handled in compliance with our privacy policy.