Sign up for our newsletter!
Your data will be handled in compliance with our privacy policy.
Your data will be handled in compliance with our privacy policy.

Fertilizer production requires large amounts of hydrogen. Today, 95 percent of this hydrogen is produced from fossil fuels, leading to colossal greenhouse gas emissions of CO₂. To save the Earth from more than 2 °C of global warming, politicians are using economic incentives to get the fertilizer industry to switch from fossil hydrogen to clean hydrogen produced by electrolyzers. This makes the fertilizer industry one of the largest markets for electrolyzers. In this blog post, we take a closer look at this little-known market, which, given its size, is more interesting than many of the more well-known ones.
Thomas Barregren • February 8, 2024
Which modern invention has meant the most to humanity? The steam engine, train, airplane, car, space rocket, nuclear power, radio, television, computer, AI, …? The list of contenders is long. But none of them can match…
Sound a fanfare, please!
…the Haber-Bosch process.
Haber and Bosch!? What have Peter Haber and Harry Bosch done for humanity? you ask in disbelief.
Well, they have entertained us. At least a few of us. Peter Haber is a Swedish actor best known for playing Martin Beck, and Harry Bosch is a fictional character in Michael Connelly’s novels. But they are, of course, not the Haber and Bosch behind the modern invention that has meant the most to humanity.
The Haber and Bosch I am talking about are the German chemists Fritz Haber and Carl Bosch. They developed a chemical process in the early 20th century that now carries their name. This process has been justifiably described as “the most important invention of the twentieth century.”
But before we dig into what the process does and why it deserves the first spot above all other contenders, we need to take a deep breath and get some context.
The breath we just took contained 78 percent nitrogen gas (N2). 78.1 percent, to be exact. That makes nitrogen (N), by far, the most common element in the air we breathe. It is also one of the most common elements in the whole universe.
Nitrogen is also one of the building blocks of life. Literally. It’s in amino acids, proteins, DNA, RNA and ATP. (The latter is the fuel that our cells run on.)
But it’s not by breathing air that your body gets the nitrogen it needs.
You get nitrogen by eating plants, or by eating animals that have previously eaten plants, or by eating animals that have previously eaten other animals that have previously eaten…
Ok, you get the picture. The food chain. The point is that plants are ultimately our source of nitrogen intake.
But how does the nitrogen get into the plants?
Simple: their roots absorb it from the soil they grow in.
But how does the nitrogen end up in the soil? you ask relentlessly.
Today, the primary source is nitrogen fertilizer that farmers spread on fields. But let’s hold off on that. We start by looking at how nature does it without the help of humans.
Enter the scene: Diazotrophs.
What?
Diazotrophs is a collective name for bacteria and other microorganisms that convert nitrogen in the air into nitrogen compounds, mainly ammonia (NH3), which plants can take up. This process is called nitrogen fixation.
Despite all the exciting information about nitrogen, you may wonder when we will get to hydrogen. After all, that’s what you’re interested in. Look no further than the chemical formula for ammonia – NH3 – and you might see where I’m going. There are three hydrogen atoms per nitrogen atom.
When plants die, bacteria and fungi sink their teeth into the remains. (Figuratively speaking, of course; bacteria and fungi have no teeth.) The same thing eventually happens to animals and humans as well. Their remains contain nitrogen compounds (proteins, DNA, RNA, and so on). Some microbes can break these down, releasing nitrogen into the atmosphere. This process is called denitrification.Nitrogen fixation and denitrification are thus part of the great circle of life, which Mufasa teaches young Simba in the movie The Lion King. This cycle is known in science as the nitrogen cycle.
Does the nitrogen cycle go by itself?
If nature is left to take care of itself, the nitrogen cycle ticks along without any problems. But as soon as humanity put the plow in the ground and started farming, the balance was disturbed.
If crops are grown in the same place over a few years, the plants take up more nitrogen from the soil than natural processes can restore. This is why humans have come up with strategies such as slash-and-burn agriculture, crop rotation, and fertilization.
The practice of fertilization dates back to ancient times, with early civilizations such as the Sumerians and Egyptians using manure to enrich soil around 2000 BCE. This early form of fertilization helped improve crop yields, showcasing humanity’s initial understanding of enhancing soil fertility for agricultural purposes.
Manure and humus have been used as fertilizers since the time of the Sumerians and the Egyptians. However, the idea of creating a synthetic fertilizer was not born until the 19th century.
In his groundbreaking book Die organische Chemie in ihrer Anwendung auf Agricultur und Physiologie, first published in 1840, the German chemist Justus von Liebig argued that nitrogen compounds, such as ammonia, were needed to grow the healthiest crops possible. This earned him the epithet “father of the fertilizer industry.”
Justus von Liebig’s theory led to a rush for nitrogen at the end of the 19th century. Saltpeter was mined with an unprecedented frenzy, and tropical rocks were scraped for guano.
But saltpeter mines and bird poop only went so far.
At the end of the 19th century, it was realized that natural sources were not sufficient to meet future needs. This sparked the idea of somehow extracting nitrogen directly from thin air.
Several methods were developed to fix the nitrogen in the air. But it was not until the beginning of the next century that the real breakthrough came, albeit with a humble beginning.
In 1905, Fritz Haber produced a small amount of ammonia by mixing nitrogen and hydrogen at 1,000 °C in the presence of an iron catalyst. However, the high temperature made the method impractical.
Over the next few years, Fritz Haber refined his technique. In March 1909, he presented a method in which the temperature had been reduced to the more manageable range of 500–600 °C. This was accomplished with high pressure. The process requires almost 200 times the air pressure (20 MPa).
But there was a caveat. The ammonia was produced drop by drop; it took 8 hours to produce a single liter of ammonia. Nevertheless, Fritz Haber had demonstrated a viable solution for extracting nitrogen from the air and fixing it as ammonia.
The German chemical company BASF purchased the rights to the process and tasked Carl Bosch with scaling up Haber’s tabletop machine to industrial scale. Four years later, the BASF factory in Oppau produced five tons of ammonia – per day.
This is why the process is named after the two men: The Haber-Bosch process.
Haber and Bosch were awarded the Nobel Prize in 1918 and 1931, respectively, for their work in solving the chemical and engineering problems of large-scale, continuous flow and high-pressure technology.

Initially, Haber-Bosch process was mainly used to produce ammonia for the military and industry, but after the Second World War, the use of ammonia as a nitrogen fertilizer in agriculture exploded.
The increased use of synthetic nitrogen fertilizer led to higher yields that supported a rapidly growing population. That’s why Professor Vaclav Smil wrote in the prestigious scientific journal Nature that the Haber-Bosch process ”is the most important invention of the twentieth century.”
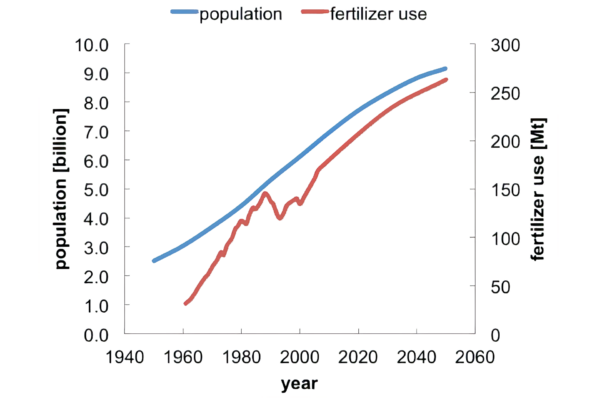
An often-quoted statistic is that nitrogen fertilizers are responsible for feeding half the world’s population.
Since this fertilizer is produced through the Haber-Bosch process by converting hydrogen, I think it’s fair to say that hydrogen feeds the world.
Don’t you agree?
But the mass adoption of synthetic fertilizers has come at a high cost to the environment: harmful algal blooms, soil acidification, and massive greenhouse gas emissions.
A study estimates that the production and use of nitrogen fertilizers, both organic and synthetic, in food-growing accounts for around 5 percent of global greenhouse gas emissions and that this could threaten efforts to keep global warming below 2 °C.
Nitrogen fertilizers contribute to the greenhouse effect in many ways.
One issue is that far from all fertilizer is absorbed by plants, and what remains is broken down by microbes in the soil, producing laughing gas (N2O).
That’s not funny at all. (Pun intended, of course.)
Laughing gas, or nitrous oxide, which is its chemical name, is a greenhouse gas almost 300 times more potent than carbon dioxide (CO2).
But another major source is the production itself. The manufacture of artificial fertilizers is responsible for almost 1.5 percent of total global CO2 emissions.
The Haber-Bosch process is carried out at high temperatures and pressure, turning the production plants into energy-hungry monsters. The energy comes from burning natural gas.
Natural gas is also used to produce gray hydrogen, which is the feedstock in the Haber-Bosch process. Much hydrogen is needed. Remember that forming ammonia takes three hydrogen atoms per nitrogen atom (NH3).
About 40 percent of the fossil gas input into the process is burned to fuel the reaction, with the remaining 60 percent being used as feedstock.
Producing ammonia fertilizers is responsible for about 1 percent of all global energy use and 1.4 percent of CO2 emissions.
More than 180 million metric tons of ammonia are produced annually. Nearly 90 percent of ammonia is used to produce synthetic nitrogen fertilizers (including urea, ammonium nitrate, and ammonium phosphate).
Producing this massive amount of ammonia requires more than 32 million metric tons of hydrogen. Today, more than 95 percent of this hydrogen is produced from natural gas and coal.
To produce 32 million tons of hydrogen by steam methane reforming (SMR), the most common method, approximately 80 million tons of natural gas are required, assuming an efficiency rate of 80 percent for the SMR process.
Thus, we can calculate that 68 million tons of natural gas are needed just as a feedstock in the production of nitrite fertilizer. The Haber-Bosch process consumes an additional 45 million tons of natural gas as fuel. In total, 113 million tons of natural gas are needed annually to produce synthetic nitrogen fertilizers.
When all this natural gas is used, more than a staggering 310 million metric tons of carbon dioxide (CO2) are released into the atmosphere.
Oops!
For hydrogen to continue to feed half the world while keeping global warming below 2 °C, the production of synthetic nitrogen fertilizers must switch from fossil-based hydrogen to fossil-free hydrogen rather quickly.
However, this requires many PEM-electrolyzers to produce the fossil-free hydrogen.
Now you understand the connection between fertilizers and Smoltek.
You were probably worried for a while, as the article took you on a seemingly outlandish journey through the history of fertilizers – from Sumerians through 1910s Germany to today. But as you now understand, there is a purpose.
Many think hydrogen will be used mainly to power cars and other vehicles. Some know there are other applications, such as storing excess electricity from solar and wind turbines. But most people miss the really big applications for electrolyzers, and one of them is replacing natural gas used by Haber-Bosch process plants.
Whether an electrolyzer produces hydrogen to feed the world or power a sports car, there is a problem.
The cell material in a PEM-electrolyzer contains iridium. Today, 2.0 milligrams of iridium are used per square centimeter. That doesn’t sound like much, but it is a lot, given that iridium is a scarce metal practically only mined in South Africa. (Some are also mined in Canada and Russia.)
The price of iridium is sky-high and expected to rise sharply with the increased demand, as it is practically impossible to extract more per year than already done.
This is why all manufacturers and buyers of PEM electrolyzers want to reduce the amount of iridium from the current 2.0 to an ambitious 0.1 milligrams per square centimeter.
Researchers and developers worldwide are working frantically to reach this dream goal. So are we. And I would say that we are ahead of the game thanks to our unfair advantage.So, if you ever had doubts about the decision to pursue the hydrogen market in parallel with the semiconductor market, it’s time to stop doubting. Don’t you agree?
Your data will be handled in compliance with our privacy policy.